The FDA has again approved a generic version of the most commonly prescribed medication for teens in the U.S. without requiring proof that the new product works as well as what kids are currently taking. The FDA took this action after withdrawing approval of the last two generic versions of Concerta approved through the same process.
When Concerta first hit the market in 2000, it revolutionized the treatment of ADHD because it offered the first truly effective method for prolonging the effects of methylphenidate (the active ingredient in Ritalin and many other stimulant preparations) so that kids no longer needed to go to the principal's office or nurses' office in the middle of the school day. When Adderall XR followed in 2001 and Strattera in 2002, a vast increase ensued in the number of children and teens identified with and treated for ADHD. Out of the roughly 25 medications currently approved for ADHD, Concerta remains very popular because of the consistency of its' effects throughout the school day and immediately after school. It is widely used in teenagers because of its' beneficial effects on driving performance.
A basic principle in understanding how extended-release stimulant products work is that the manner in which the drug is released into the body (the drug delivery system) has profound effects on the pharmacodynamics (the observed benefits/response to the drug). We have a number of approved medications for ADHD in which methylphenidate is the active ingredient. In addition to Concerta, Ritalin LA, Metadate CD, Daytrana, Quillivant and Focalin XR are all extended-release methylphenidate products. The effects of the product at specific times throughout the day result from differences in how the medication is released and absorbed into the body with each unique delivery system, and form the basis of how we decide which product we choose for an individual child. Allow me to illustrate...
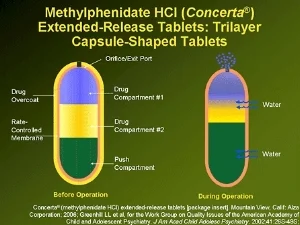
Concerta utilizes something called the OROS delivery system to release methylphenidate into the body. It was developed by a team of scientists in California who observed a phenomena referred to as "tachyphylaxis" with earlier attempts to develop long-acting methylphenidate-based stimulants. Essentially, people taking stimulants develop some degree of tolerance to the drug acutely in response to an individual dose. While the absolute level of the drug in someone's system matters, a rising blood level is often necessary to sustain the beneficial effects of medication over the course of a school or work day.
Concerta was designed to release an initial dose of stimulant within the first two hours of ingestion...22% of the active drug is contained within the coating of the pill. After this overcoat dissolves, a laser-drilled hole in the end of pill is uncovered. As the pill passes through the stomach and the gastrointestinal track, water taken up into the pill results in changes in internal pressure that leads to a "pulse release" of small amounts of medication as it passes through the gut. The effects of Concerta were tested in a laboratory classroom setting, in which raters blinded to whether kids received active drug or placebo scored the observable behavior of kids throughout a twelve hour day, and an age-appropriate mini-math test (PERMP) was administered at intervals throughout the day to measure medication effects on cognitive performance. The results are pictured below. A significant benefit of Concerta is the consistency of improvement in cognitive performance throughout the day.
In contrast, Focalin XR utilizes a "beaded" delivery system to release methylphenidate into the body. Focalin XR is a capsule containing two types of beads. The outer coating of the capsule dissolves very quickly (within ten minutes) upon ingestion. 50% of the beads inside Focalin XR release almost immediately after the coating of the outer capsule dissolve, while another 50% have a different coating designed to dissolve approximately four hours after the capsule is swallowed. One advantage of Focalin XR is that parents can crack open the capsule and sprinkle the contents in yogurt or applesauce when kids can't swallow pills, whereas Concerta won't work if the pill isn't swallowed intact. Another advantage with 50% of the medication released immediately is that the medication kicks in very quickly in the morning with demonstrable benefits at 30 minutes (see below). In practice, Focalin XR has a pronounced peak effect in late morning and is very effective for most kids throughout the school day. At the same time, the cognitive effects of the drug fall off much more quickly during the latter part of the day compared to Concerta, and drug company marketing claims aside, I find in my patients that Focalin XR is a good choice for kids who need medication to cover the duration of their school day, but not much longer.
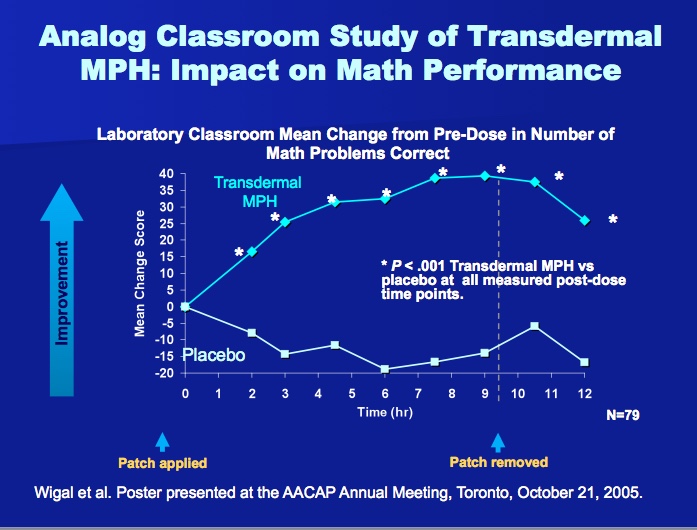
Daytrana is a patch worn on the hip in which methylphenidate is absorbed through the skin as a result of an osmotic gradient...the difference in the concentration of methylphenidate in the patch vs. the difference in the concentration of methylphenidate in the capillaries supplying blood to the skin. A unique benefit of Daytrana is that it will last longer than any of the other stimulant products on the market...it was originally developed to be a 16-18 hour drug. Because the testing required for approval by the FDA examined the effects of the product over a twelve hour period, the company that manufactures Daytrana isn't permitted to share that information with prescribers. The cognitive effects of Daytrana also peak later in the day than with other products (see below), making Daytrana very helpful for many kids who struggle with homework after school. Comparing the laboratory classroom studies of Daytrana to Concerta and Focalin XR, an obvious downside to Daytrana is that it doesn't work as well during the first half of the school day. Many parents resort to either putting the patch on their child very early in the morning while they're still sleeping to overcome this effect, or give their child a small dose of immediate-release methylphenidate (Ritalin or immediate-release Focalin) when they first apply the patch on in the morning.
My point is that what makes Concerta work like Concerta is the OROS delivery system. The same drug (methylphenidate) released through a different delivery system produces a VERY different response.
The arrival of generic Concerta was delayed for a number of reasons...the makers of Concerta fought the lawsuits of the generic manufacturers aggressively, and pursued a legal strategy involving what's referred to as a "Citizen's Petition" requiring generic companies seeking to copy Concerta to demonstrate a similar pattern of ascending blood levels throughout the day. Because Johnson & Johnson (the parent company that owned the rights to Concerta) owned the patent on the OROS release system, companies seeking to make a generic version had to do so with a different delivery system.
Ultimately, Watson Pharmaceuticals (subsequently acquired by Actavis) was approved to manufacture a generic equivalent of Concerta. As often occurs in these situations, the lawyers for Johnson & Johnson and Watson worked out a deal to avoid years of legal battles in which J & J would continue to manufacture Concerta through their Alza subsidiary that Watson would sell at a discount as an "authorized generic," with the two companies splitting the profits. The brand Concerta and the Activis version of Concerta are equivalent...they are manufactured in the same factory, using the same equipment and the same drug delivery system as in the original Concerta. Pictures of the "authorized generic" using the OROS system are shown above:
The next two versions of versions of generic Concerta (manufactured by Mallinkcrodt and by Kremers Urban) each used very different drug delivery systems (release mechanisms) in an effort to replicate the therapeutic effect of Concerta.
In the case of the Mallinkcrodt product, an overcoat containing immediate-release methylphenidate that dissolves within the first hour after ingestion. The core of the pill contains a diffusion-controlling membrane that releases methylphenidate as water in the gastrointestinal tract passes through the membrane. The membrane is designed to release methylphenidate over a period of time roughly corresponding to the release period resulting from the OROS delivery system in Concerta.
The Kremers Urban generic uses an extended-release bead technology to release methylphenidate at a controlled rate. The pill resembles a conventional tablet in appearance, featuring an overcoat containing immediate release stimulant that releases during the first hour as the tablet disintegrates and a core of extended-release stimulant beads operating with a similar mechanism as those in Focalin XR.
Here are links to the FDA-required product information or "labels" for Concerta, the Mallinkcrodt generic version and the Kremers Urban generic version that are being substituted for Concerta. It appears that the FDA allowed the generic manufacturers to "cut and paste" the data from Concerta's pharmacokinetic studies and clinical trials and present this information as if it represented trials each company conducted with their own unique product.
The absorption of the original Concerta depends to some degree on an individual's GI transit time...i.e., how long it takes for the pill to pass through the gut. Bead release systems (as in the Kremers Urban version) typically depend upon the acidity of the contents of the stomach at the time the extended-release bolus of medicine is needed. One would anticipate an individual child or teen might absorb significantly more (or less) medicine at different times during the day when two products that on average deliver roughly the same amount of medication over the same time period depend upon different physiologic processes.
When the FDA requires generic companies to do studies demonstrating "equivalency" to a brand medication, the amount of medication taken up into the body (measured by what we refer to as the "area under the curve" or AUC) is required to be within 80-125% of that observed with brand name drug. With some types of medication, that variability makes little difference. With stimulants, small differences in either the rate at which the medicine is absorbed or the time at which the medicine is absorbed make a PROFOUND difference in the benefits or side effects experienced by an individual child or adult. The FDA doesn't require generic companies to conduct comparison studies showing that the products work as well in practice as the brand name drugs they're intended to replace. Neither Mallinkcrodt nor Kremers Urban was initially required by the FDA to conduct a study showing that their drug works as well in practice as the brand or authorized generic versions of Concerta.
Restating my earlier point, what makes Concerta work like Concerta is the OROS delivery system. The same drug (methylphenidate) released through a different delivery system produces a VERY different response.
Ultimately, a small, randomized study was published in Clinical Pediatrics demonstrating the superiority of the "brand" Concerta and generic Concerta using the OROS delivery system marketed by Actavis, compared to the Kudco and Mallinckrodt versions of generic Concerta using non-OROS delivery systems. In response to this study and consumer complaints, the FDA demanded that each company submit additional data demonstrating their products to be equivalent to Concerta. Mallinckrodt did not comply with the request, while data submitted by Kremers Urban was deemed insufficient.
Given the calls that have started coming into my office over the last day or so, there's a very good possibility that a similar scenario will play out again as the newest generic version of Concerta, manufactured by Osmotica through their Trigen subsidiary hits the market this month. The FDA again allowed a generic manufacturer (Osmotica) to substitute the data from Concerta's clinical trials in their product-specific information, instead of requiring them to publish pharmacokinetic data specific to their product.
The drug delivery system being used with the Trigen system is more similar to the OROS system used in Concerta, but it's still different. Families will also face tremendous pressure to switch to the new product because the FDA approved a 72 mg version of the Trigen generic (the most common Concerta dose in teenagers) because insurance companies and pharmacy benefit managers will save money by paying for half as many pills as are currently required. I hope the Trigen version works as well as Concerta. It might be more potent. It might be less potent. We have no more way of knowing how it will work than we did with the Kremers Urban and Mallinckrodt products for which approval was withdrawn by the FDA.
The last time the pharmacies began switching kids to cheaper generic versions of Concerts, I had over ten kids show up in my office reporting a significant decline in the effectiveness of their medication. I wrote much of this post after seeing a mother and her daughter who reported problems with medication when the appearance of her pill changed. I sent them back to the drugstore with new prescriptions for the brand Concerta or the authorized generic version. The pharmacist told the mother it was illegal for them to fill the prescription for the original product (not true) even though she was willing to pay for the prescription "out of pocket" and threatened to call the police if she insisted on having my prescription filled.
I encourage families of kids who are taking Concerta to be prepared on your next trip to the pharmacy. If you have no other affordable option, make a point of informing your child's prescriber and the FDA if they don't respond as well to the new generic or experience more side effects on the new generic.
***********************************************************************************************************
Key Ministry has assembled a helpful resource page for church leaders and parents addressing the topic of ADHD and spiritual development. This page includes our blog series on the topic and links to helpful videos and resources for pastors, church staff, volunteers and parents. Access the resource page here.